Are you reading this article on a cell phone? Maybe you’re on the wifi at a local coffee shop. Well, this article, and anything else you browse while waiting for your frappuccino, is delivered to your mobile device thanks to a 1940’s Hollywood starlet. Digital communications wouldn’t exist without the elegant brilliance of Hedy Lamarr, another prominent woman in science.
 Austrian born, Hedy Lamarr debuted in America opposite Charles Boyer in the 1938 film Algiers [1]. She became a sensation thanks in large part to her beauty, working with Hollywood headliners like Clark Gable, Jimmy Stewart, and Judy Garland. Her biggest success was her titlular role in Samson and Delilah, the highest grossing film of 1949 [2]. But Hollywood started to typecast Hedy as the seductress—roles that required very little beyond looking pretty. Bored with her acting career, Hedy switched to inventing [3].
Austrian born, Hedy Lamarr debuted in America opposite Charles Boyer in the 1938 film Algiers [1]. She became a sensation thanks in large part to her beauty, working with Hollywood headliners like Clark Gable, Jimmy Stewart, and Judy Garland. Her biggest success was her titlular role in Samson and Delilah, the highest grossing film of 1949 [2]. But Hollywood started to typecast Hedy as the seductress—roles that required very little beyond looking pretty. Bored with her acting career, Hedy switched to inventing [3].
Her first few inventions were flops, including a tablet to automatically carbonate water (which Hedy admitted made the drink taste like alka seltzer) [3]. Then she focused on military inventions to help the Allies in WWII. At a dinner party, Hedy met composer George Anthiel, and chatted about radio communications used to control torpedoes [1]. These signals could easily be intercepted or jammed by the enemy, but Hedy realized that randomly changing the frequency of the transmission might make radio communications harder to decipher. The two started working on a “frequency-hopping” procedure they later patented [4].

Source: Krzysztof S pl / Wikimedia Commons
Hedy and George’s ‘frequency-hopping’ system was inspired by paper piano rolls, where up to 88 perforations in a roll of paper control which keys are played on self-playing pianos. But instead of playing a song, the paper rolls would control the frequency of the radio message. Transmitting and receiving stations would synchronize identical paper rolls, allowing communications to hop between frequencies seemingly at random [4]. With the transmitter and receiver hopping frequencies to the tune of Camptown Races, the Nazis wouldn’t be able to lock in on a signal to intercept communications or jam the frequency.
Hedy and George’s invention was never used in WWII, but it was adopted for military communications during the Cuban Missile Crisis. Later, Hedy and George’s invention would form the basis of secure digital communications via satellite, wifi, and cellular phones [5].

Each of these mobile devices trades something akin to an electronic piano roll, to sync their communications and prevent interference between signals.
This technology wasn’t widely adopted until after their patent expired, so Hedy didn’t strike it rich in the digital age. But she was recognized for her invention in 1997 with an Electronic Frontier Foundation (EFF) Pioneer Award and was the first female recipient of a BULBIE Gnass Spirit of Achievement Award [5].
Hedy once said “Any girl can be glamorous; all you have to do is stand around and look stupid” [1]. Lucky for the internet, it was all an act.
References
[1] “Hedy Lamarr Biography“. Biography.com. Retrieved 22 May 2016.
[2] Barton, Ruth (2010). Hedy Lamarr: The Most Beautiful Woman in Film. University Press of Kentucky. ISBN 9780813126104.
[3] “‘Most Beautiful Woman’ By Day, Inventor By Night”. NPR. 22 November 2011.
[4] “Patent 2,292,387“. United States Patent and Trademark Office. Filed 10 June 1941. Retrieved 22 May 2016.
[5] “Hedy Lamarr – Invention of Spread Specturm Technology“. Famous Women Inventors. Retrieved 22 May 2016.





 In Ian Fleming’s novels, all 00 agents are issued cyanide capsules in case of enemy capture, but James Bond threw his away. What a dummy. Sure, Bond always escapes, but why risk it? The rest of his peers understood their orders and were ready to die to keep their country’s secrets. Cyanide pills aren’t mere tropes either, but a real spy tool – a gruesome last resort to keep information out of enemy hands [1]. A lethal dose of cyanide kills in minutes – so effective because it blocks the body’s ability to make energy, via this week’s gene of interest: ATP synthase gamma subunit (ATP5C1).
In Ian Fleming’s novels, all 00 agents are issued cyanide capsules in case of enemy capture, but James Bond threw his away. What a dummy. Sure, Bond always escapes, but why risk it? The rest of his peers understood their orders and were ready to die to keep their country’s secrets. Cyanide pills aren’t mere tropes either, but a real spy tool – a gruesome last resort to keep information out of enemy hands [1]. A lethal dose of cyanide kills in minutes – so effective because it blocks the body’s ability to make energy, via this week’s gene of interest: ATP synthase gamma subunit (ATP5C1).

 People can’t live without vitamin C, but everyone’s heard a story about that one college student who tried to live off of nothing but chips and ramen [1]. And were it not for a mutation a few millions of years ago, the story wouldn’t end in the clinic with a diagnosis of scurvy. We could synthesize all the vitamin C we need with a few repairs to this week’s gene of interest: L-gulono-γ-lactone oxidase or GLO.
People can’t live without vitamin C, but everyone’s heard a story about that one college student who tried to live off of nothing but chips and ramen [1]. And were it not for a mutation a few millions of years ago, the story wouldn’t end in the clinic with a diagnosis of scurvy. We could synthesize all the vitamin C we need with a few repairs to this week’s gene of interest: L-gulono-γ-lactone oxidase or GLO.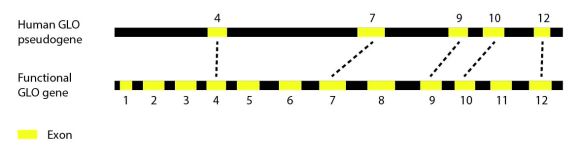
 So that’s why you should eat your fruits and veggies – because evolution has failed you. But considering the American diet, it’s probably a good thing we can’t make our own vitamin C. If not for a horrifying death by scurvy, too many people would opt to avoid anything green for tasty, tasty junk food.
So that’s why you should eat your fruits and veggies – because evolution has failed you. But considering the American diet, it’s probably a good thing we can’t make our own vitamin C. If not for a horrifying death by scurvy, too many people would opt to avoid anything green for tasty, tasty junk food.