artwork by Molly Fischer
The sketches at the top of this post belong to my talented former student, Molly Fischer. She has a knack for pulling beauty from subjects that may at first seem clinical or even macabre. But I agree with her that bones tell a beautiful story. Sort of like Stonehenge: a mystery structure leaving clues to what was.
Much of our natural history is told through skeletons – from the dinosaurs to early Homo sapiens. But bones can tell us more than just the size and shape of something long gone. Bones are alive (at least while we’re alive) so they hold clues to how that life was lived. They are an amazing material that grows and adjusts in response to external forces. And the protein responsible for bone’s adaptability is made by this week’s gene of interest: sclerostin or SOST
Bones are in a constant state of flux – broken down and rebuilt over and over [1]. Breakdown is done by cells called osteoclasts; rebuilding, by osteoblasts. All things being equal, bone will be rebuilt in the same way, never changing structure. That makes this sound like a useless process, but when there’s a change, constant rebuilding allows the bone to adapt to new forces in the environment.
For example, in space, bone density drops because there are almost no external forces. Astronauts lose about 1% of their bone mass for each month spent in zero gravity, so bone loss will be a huge obstacle for long-term space travel [2]. But bone density can increase too. Many martial arts, including the style that I train (Goju-ryu), regularly condition their bodies to strengthen their bones. Sensei Morio Higaonna, a 10th degree black belt in Goju-ryu, regularly strengthens his hands by pounding his fists on a boulder [3]. With each hit, he strengthens the bones in his hands, effectively transforming his fists into boulders. See below for a video of this sophisticated training technique. And please, talk to your sensei before trying this at home.
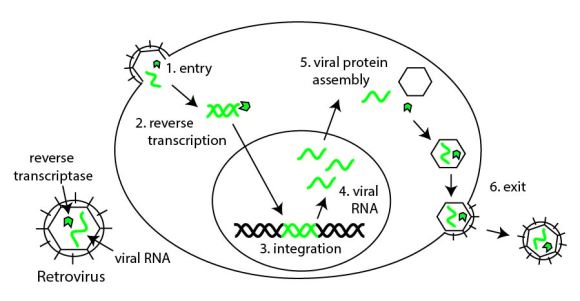
So how can bone feel force? Bone is embedded with cells called osteocytes. They’re woven through bone like little spider webs [1]. These cells constantly make a protein called sclerostin (from the SOST gene). Osteocytes function as microscopic strain gauges. When they feel force, they stop making sclerostin. This tells the osteoblasts and osteoclasts on the surface to build stronger bone on their next cycle.
Osteoclasts and osteoblasts constantly breakdown and rebuild bone, while osteocytes make sclerostin. (1) When a force is applied, (2) the osteocytes stop producing sclerostin, (3) which signals to the osteoblasts to make stronger bone. (4) After the bone is remodeled, osteocytes start making sclerostin again.
So sclerostin functions as an “everything’s OK” alarm. No force: plenty of sclerostin. Sensei Higaonna pounds his fist into a boulder: sclerostin turns off; the body builds denser bone until the force is no longer felt.
If small bits of force, from running, lifting weight, punching boulders, create strong bones then it should come as no surprise that modern bones are weaker than those of our ancestors [4]. After the introduction of farming about 7,000 years ago, people became more sedentary and their bones reflected that change. Bones steadily lost density, especially in regions on the legs associated with running and walking long distances. So bones record our history, activity level, maybe even changes in our diet. What story will your bones tell?
References
[1] Bonewald, L. (2011). “The Amazing Osteocyte”. J Bone Miner Res. 26(2): 229–238. PMC 3179345.
[2] “Bone Remodeling”. Wheeless’ Textbook of Orthopaedics. 13 September 2011.
[3] “Morio Higaonna’s 3 Ultimate Lessons of Karate Wisdom”. Karate by Jesse.
[4] “Researchers Discover Why Our Ancestors Had Stronger Bones”. Newsmax. 18 May 2015.



 We’re still not sure how salamanders do this, but stem cells may be the key. When salamanders lose a limb, cells at the site of amputation reprogram themselves into adult stem cells [1]. These cells have the potential to turn into many different cell types, a property called multipotency. Cells at the amputation site rapidly divide and form muscle, bone, and skin to replace the lost limb. Humans also have adult stem cell populations that replenish red and white blood cells and participate in limited healing, but regrowing a human limb remains just out of reach (Amputation puns are disarming).
We’re still not sure how salamanders do this, but stem cells may be the key. When salamanders lose a limb, cells at the site of amputation reprogram themselves into adult stem cells [1]. These cells have the potential to turn into many different cell types, a property called multipotency. Cells at the amputation site rapidly divide and form muscle, bone, and skin to replace the lost limb. Humans also have adult stem cell populations that replenish red and white blood cells and participate in limited healing, but regrowing a human limb remains just out of reach (Amputation puns are disarming).